Main navigation
Microalgae: sustainable chemical production in a mini factory
Renewable raw materials that can be used as alternatives to fossil resources already exist. However, to turn them into everyday products, plant oils and other renewable raw materials not only have to be extracted, but often have to undergo complex chemical processing. Researchers at the University of Konstanz have now converted microalgae cells into tiny refineries to produce and upgrade raw materials, and thus create a supply of sustainable chemicals.
It has long been known that our fossil-based raw material sources are finite, and the search for alternatives has been going on for almost as long. It has been quite successful, because there is already a whole range of renewable resources - such as wood, natural fibres and vegetable oils - that can be used for producing chemicals or fuels just like their fossil counterparts. With the difference that they cannot be used directly, because they are raw materials that need to be refined, i.e. extracted and separated from the cells before they can be upgraded and further processed. These are time-consuming and costly steps that mean the products derived from them are not yet truly competitive.

Suitable refinery solutions are therefore needed to make renewable raw materials an equivalent alternative to fossil-based raw materials. That is why Prof. Dr. Stefan Mecking and his research group at the Department of Chemistry at the University of Konstanz have been working on the chemical conversion of biomass for over ten years. One of the objects of research is the microalga Phaedodactylum tricornutum, a diatom that is photoautotrophic, i.e. it only needs sunlight, CO2 and water - preferably salt or brackish water - to grow and produce energy, thus using no arable land or freshwater resources. It grows robustly and quickly and has a high unsaturated fatty acid content, so the researchers are using it as a raw material for producing algae oils.
Fatty acids of this kind are currently mainly obtained from palm, soy, rapeseed or sunflower oil and are among the most important renewable raw materials for the chemical industry: in Germany, for example, around half of the renewable raw materials used in recent years have been oils and fats, which have been used for producing surfactants, cosmetics, polymers and adhesives, among others.1)
Nature is tricked into introducing synthetic catalysts into living cells
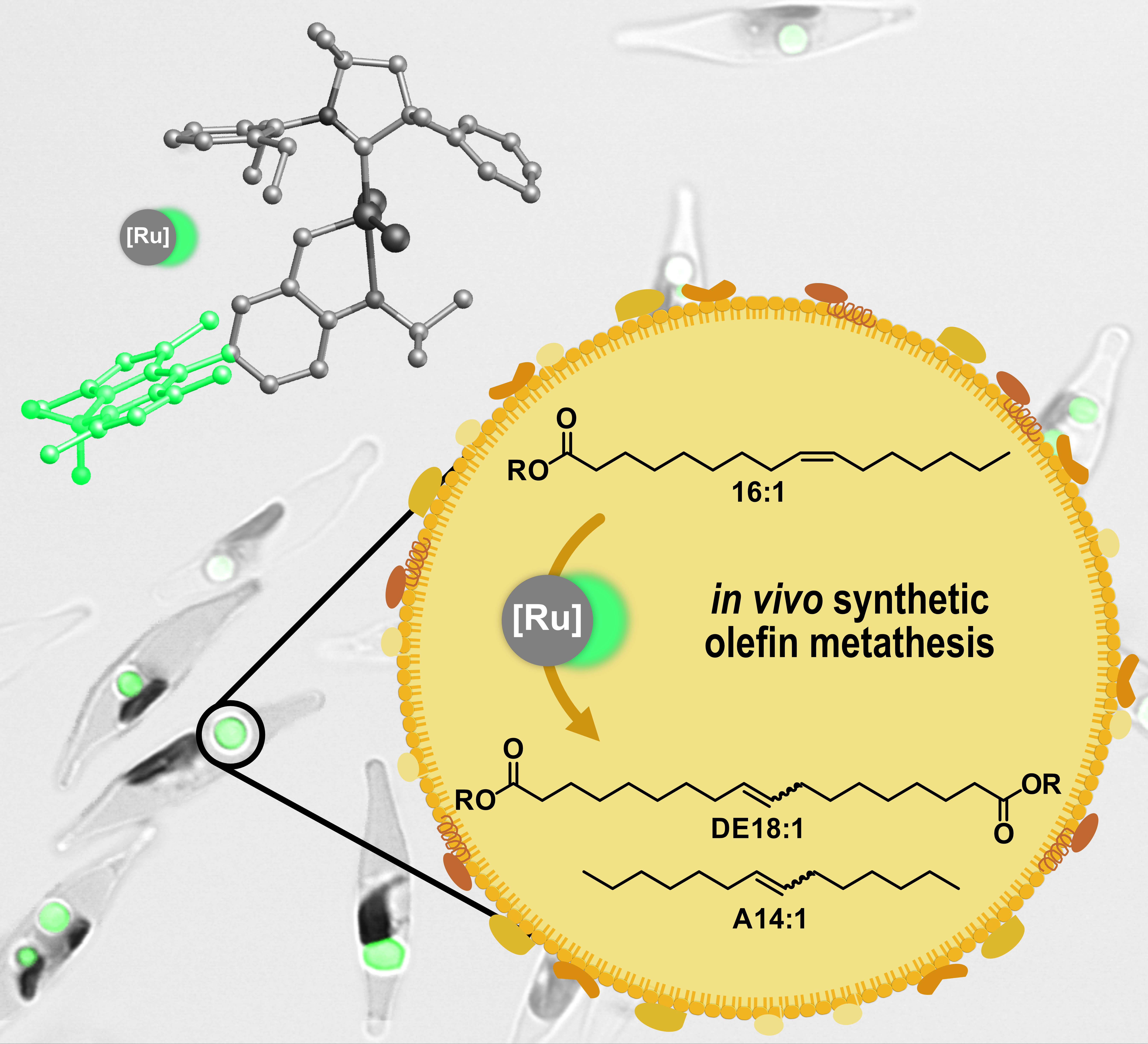
These oils and fats are converted into the desired products - such as long-chain alkenes or dicarboxylates - with the help of what is known as olefin metathesis, an organic reaction that entails the reorganisation of the carbon-carbon double bonds of the unsaturated fatty acids in the presence of a catalyst. "This works pretty well, at least in principle. However, the prior extraction of the unsaturated fatty acids from biomass, which consists of a broad range of different compounds, usually creates a bottleneck," Mecking reports. "And since we mainly deal with catalytic upgrades, we asked ourselves whether olefin metathesis could take place directly in the cell. It isn’t that easy, as these are reactions that the cellular machinery is not naturally capable of and no natural biocatalyst is known either."
Chemist Natalie Schunck, a doctoral student in Mecking's team, took on this challenge and managed to find a way to introduce synthetic catalysts into the diatoms where they are needed, namely in the lipid bodies where the microorganisms produce and store fats. "This was a very challenging project", the professor stresses. "The project was only successful thanks to the outstanding work done by Ms. Schunck who brought to the project a solid background in chemistry as well as proven biological expertise."
Sustainable materials produced in living cell factories
The fact that it is far from easy to smuggle the catalyst into the mini factory and start working is down to the anatomy of the microalgae cell: it has a robust cell wall that first has to be overcome, then, in order to make its way from the cell wall to the lipid storage compartments, the catalyst has to get across the aqueous cytoplasm that contains components that might be harmful to the intruder. Schunck only succeeded through trickery. She coupled a synthetic ruthenium-based catalyst to a lipophilic fluorescent dye that is normally used to stain lipid bodies in algae cells. This ensured that the catalyst safely reached the lipid storage compartments where it could then catalyse the desired conversion reaction.2)
"The dye has two functions: it acts as a catalyst transporter, and we can also use it to precisely track the catalyst’s position in the cell," Mecking explains. "We found that all unsaturated fatty acids are converted in the lipid droplets, as happens, for example, with vegetable oils outside the cell in reactors. We also carried out other work in parallel in which we used the products to manufacture fully recyclable plastics similar to those made of polyethylene. But other products are also conceivable - for example in the lubricant sector - because it is possible to control which fatty acid spectrum the algae produce by genetically manipulating them, among other things." This appears to be a promising perspective for producing chemicals from living cells, which in the long term could avoid the costly extraction of biomass.
The goal is to have a continuous stream of products out of the bioreactor
This is the first time that such a synthetically catalysed reaction has ever been successfully performed in plant cells. "Other researchers have already worked on it but using different cells – e.g. bacterial cells of Escherichia coli – that do not possess a cell wall that needs to be overcome," Mecking reports.
The team’s next step is to develop a way to be able to isolate the converted raw materials from the living cells in a continuous process. "So far, we have not isolated these products from intact cells. We have extracted them, but this destroys the cells," says the chemist. "But our goal is to continuously extract the desired products from the microalgae - like in a biofactory. The key step to do this has been established, but the problem as a whole has not yet been solved. However, we definitely already have concrete ideas on how it can be done."
References:
1) Biermann, U. et al. (2021): Fatty Acids and their Derivates as Renewable Platform Molecules for the Chemical Industry. Angewandte Chemie, https://doi.org/10.1002/anie.202100778
2) Schunck N. and Mecking, S. (2022) In vivo Olefin Metathesis in Microalgae Upgrades Lipids to Building Blocks for Polymers and Chemicals. Angewandte Chemie; https://doi.org/10.1002/anie.202211285